Under the Building Capacity to Implement Rapid ART Start for Improved Care Engagement in Ryan White HIV/AIDS Program initiative 14 clinical sites across the United States were funded to implement and evaluate “Rapid Start” antiretroviral therapy (ART) services. The Health Resources and Services Administration’s Ryan White HIV/AIDS Program (RWHAP) Part F–Special Projects of National Significance (SPNS) Program sponsored this initiative to accelerate the initiation of ART and entry into HIV medical care for people with HIV who are newly diagnosed, new to care, or out of care. Each of the implementation sites had the capacity and infrastructure to support Rapid Start ART, had initiated pilot projects, or were ready to expand Rapid Start ART services, with the goal of replicating and expanding successful Rapid Start ART models. Implementation sites included RWHAP-funded clinics, Federally Qualified Health Centers, academic medical centers, and community-based organizations. Funding from the Department of Health and Human Services (HHS) Minority HIV/AIDS Fund (MHAF) also supported this initiative.
Sites | Key Components | Goals | Learning Collaborative | Evaluation | Dissemination & Replication | References
Implementation Sites
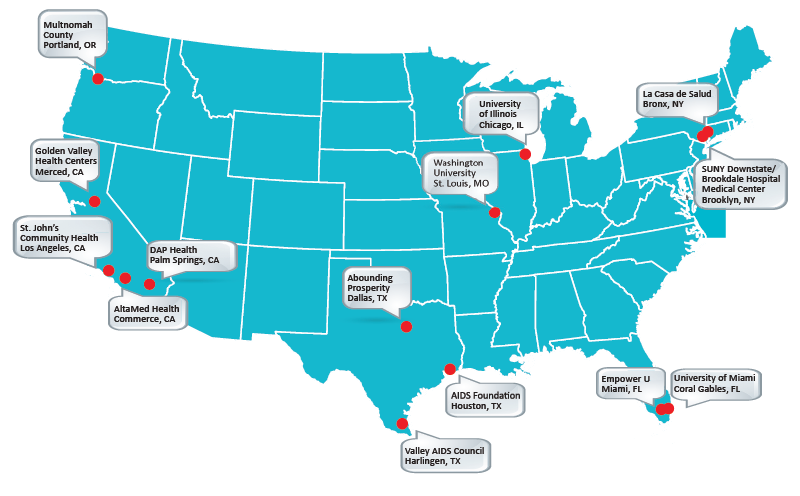
| Site | Location |
| University of Illinois | Chicago, IL |
| Abounding Prosperity | Dallas, TX |
| Valley AIDS Council | Harlingen, TX |
| AIDS Foundation | Houston, TX |
| AltaMed | Los Angeles, CA |
| St. John's Well Child and Family Center | Los Angeles, CA |
| Golden Valley Health Centers | Merced, CA |
| Empower U | Miami, FL |
| La Casa de Salud | New York City, NY |
| University of Miami | Miami, FL |
| SUNY Downstate | New York City, NY |
| Desert AIDS Project | Palm Springs, CA |
| Multnomah County Health Department | Portland, OR |
| Washington University | St. Louis, MO |
Key Characteristics of Rapid Start ART Services
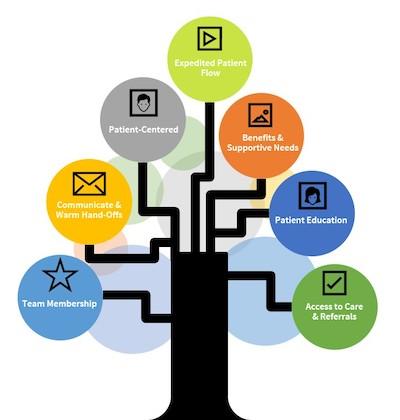
- Provision of client-centered services
- On-site testing or strong partnerships with testing programs
- Warm hand-offs and accessible linkage coordinators
- Accessible education on beginning ART
- Accelerated access to a medical visit with an HIV provider
- Early and sustained access to ART
- Pre-approved ART regimens and starter pack of medications
- Accelerated insurance/payor approval and clinic enrollment
- Follow-up with continued education, patient navigation, and supportive services
Project Goals
The University of California San Francisco (UCSF) was the initiative’s Evaluation and Technical Assistance Provider (ETAP). UCSF offered technical assistance to the implementation sites, evaluated the implementation of Rapid Start ART services, assessed HIV care continuum outcomes, and produced resources for future replication and scale-up of Rapid Start ART in other RWHAP provider organizations.
Learning Collaborative

All sites participated in the initiative-wide Learning Collaborative1 that provided the structure for technical assistance. The Learning Collaborative also provided a common set of strategies for adoption, uptake, and integration of Rapid Start ART services. Sites participated in regular initiative-wide learning sessions and received individualized coaching to support local quality improvement projects to benefit implementation outcomes. The Learning Collaborative:
- Facilitated capacity building by providing trainings, offering TA, and facilitating peer-to-peer learning to share best practices and lessons learned
- Collaborated on the development of quality measures that were used by all sites to assess progress in short cycles associated with quality improvement and longer-term trends
- Provided opportunities for engagement with subject matter experts to guide the development and implementation of Rapid Start ART
Multi-site Evaluation Focusing on Implementation, Effectiveness and Cost
Based in the Proctor Implementation Science Framework2 and Dynamic Capabilities Model,3 UCSF is using an Effectiveness-Implementation Hybrid Type 24 Design to assess the key implementation strategies for uptake of a rapid start intervention and assess HIV Care Continuum outcomes for the patients served by these 15 implementation sites. Using a mix of qualitative and quantitative data approaches (Embedded Experimental Model Design5), the ETAP multisite evaluation work includes:
- Conduct a process evaluation to characterize adaptation, implementation and barriers and facilitators to factors associated with Rapid Start intervention implementation
- Evaluate multi-site patient outcome data, including early entry into care, initiation of ART, rates of engagement, and retention in care after rapid ART initiation
- Assess the impact of adaptation and implementation of Rapid Start interventions on increased viral suppression (VS), shorter time to VS and/or durable and sustained VS
- Collect cost data to determine the labor, programmatic and structural costs associated with adapting and implementing the Rapid Start models and interventions at the implementation sites
Dissemination and Replication
Implementation materials are being developed through the course of the initiative, building to the capstone Rapid Start ART Replication & Implementation Manual. Other products include:
Other products could include:
- Materials that focus on overcoming specific challenges (e.g., marketing, integration with housing programs, integrating technology)
- Webinars and conference presentations about Rapid Start ART implementation
- Examples of clinic/agency protocols and workflow maps
- Journal articles describing evaluation findings
- Partners: Impact Marketing + Communications and TargetHIV
References
- Wells, S., Tamir, O., Gray, J., et al. (2018). Are quality improvement collaboratives effective? A systematic review. BMJ Quality & Safety, 27, 226-240.
- Proctor, E.K., Powell, B.J. & McMillen, J.C. (2013). Implementation strategies: recommendations for specifying and reporting. Implementation Science, 8, 139.
- Helfat C.E. et al.(2007). Dynamic Capabilities: Understanding Strategic Change in Organizations. Malden, MA: Blackwell.
- Curran, G. M., Bauer, M., Mittman, B., Pyne, J. M., & Stetler, C. (2012). Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical Care, 50, 217–226.
- Creswell, J.W. and Plano Clark, V.L. (2011). Designing and Conducting Mixed Methods Research. 2nd Edition, Sage Publications, Los Angeles.